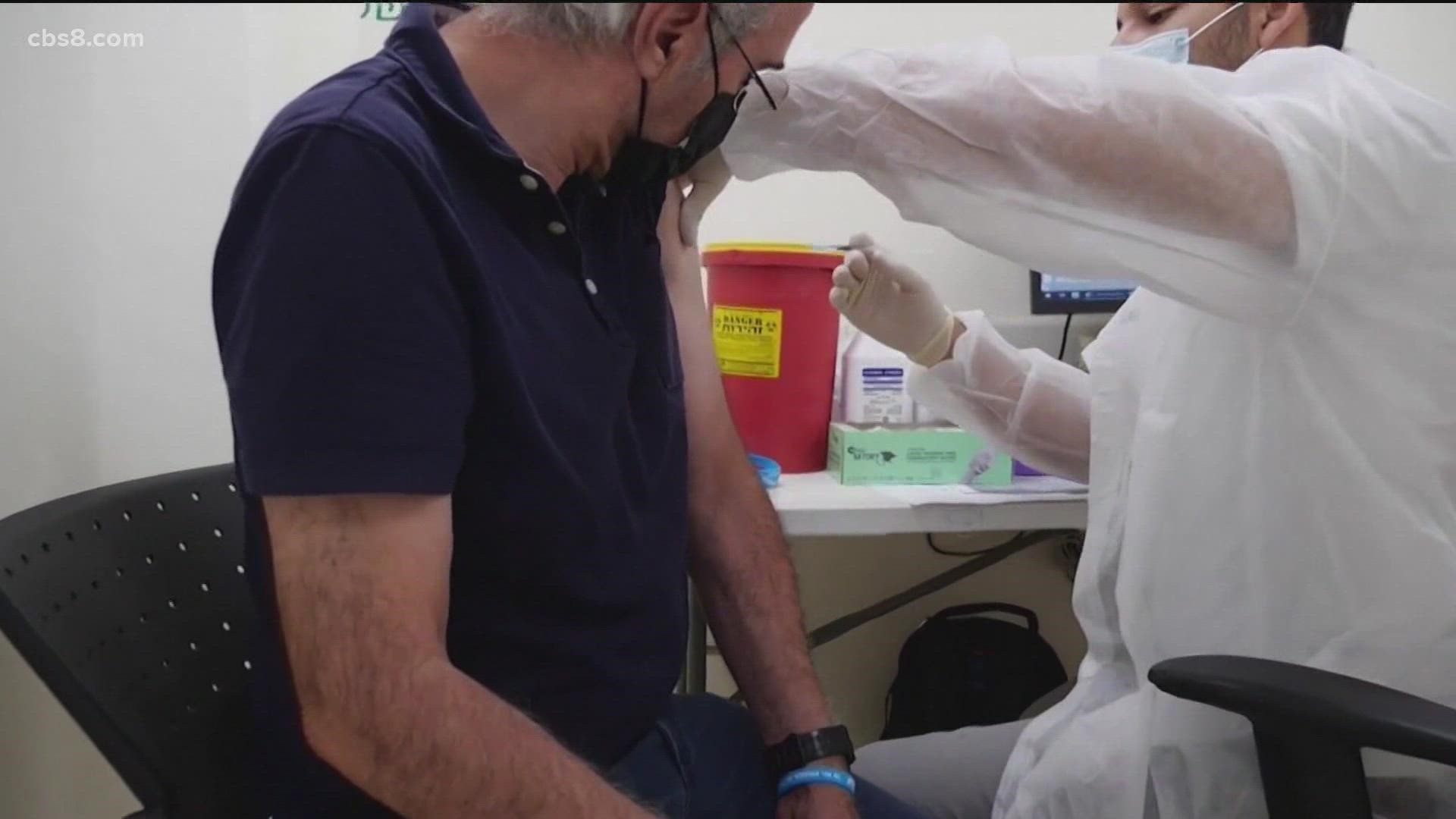
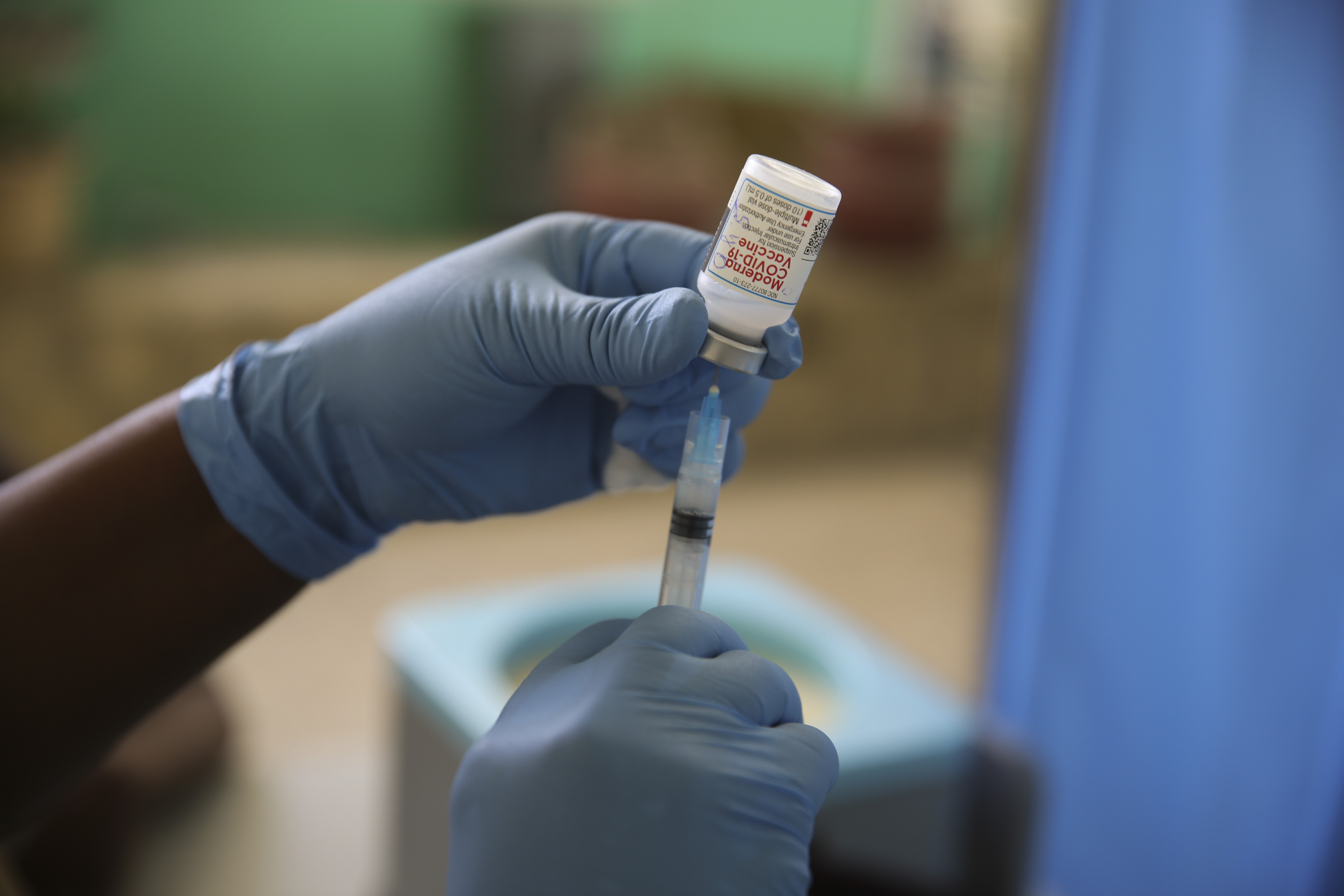
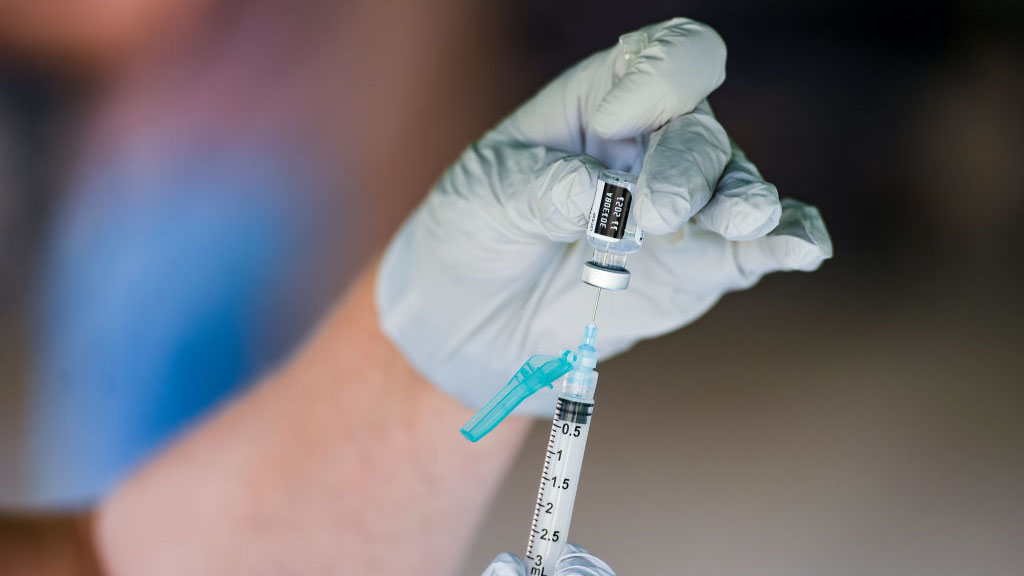
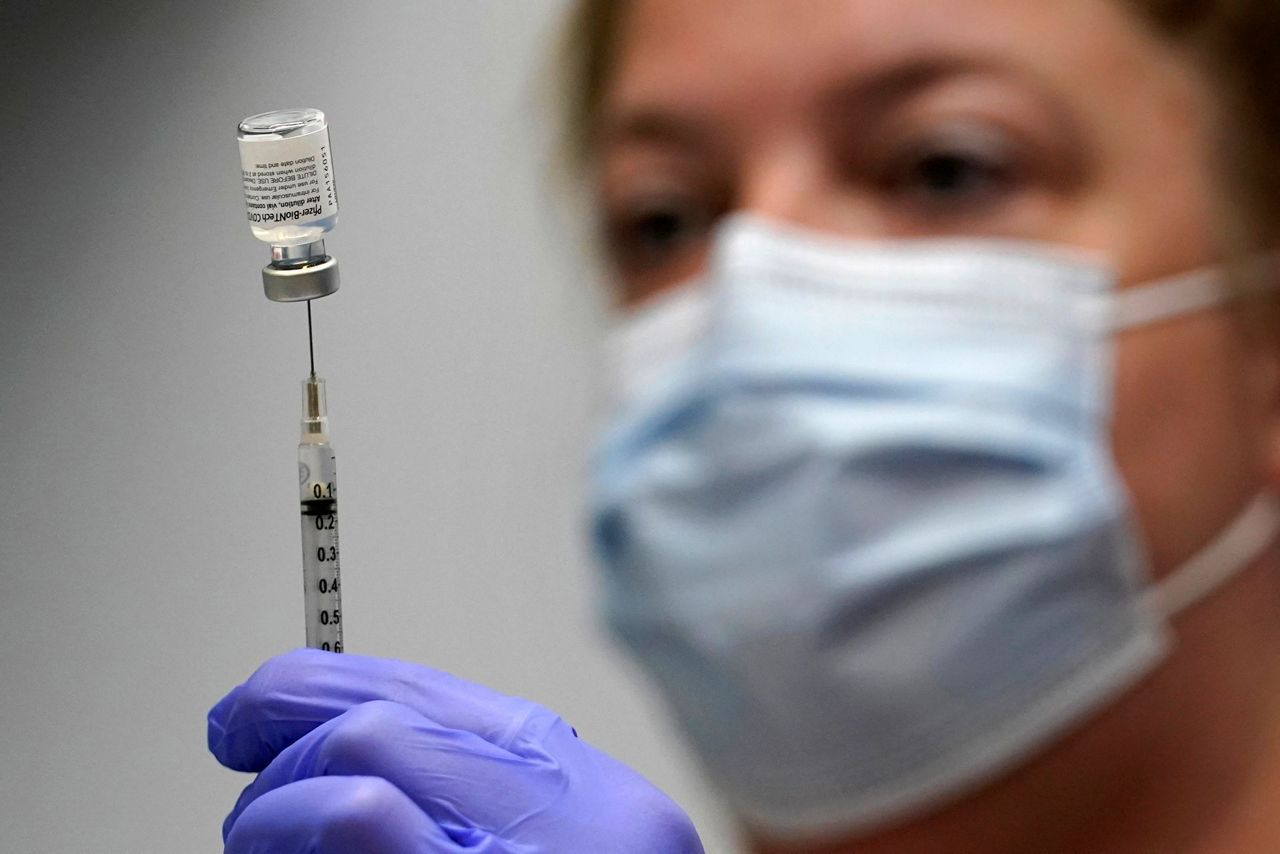
Stage 2 includes over-50s and. Post-exposure immunization Post-exposure efficacy of Imovax Rabies vaccine was successfully proven during clinical experience in Iran in which six 10 mL doses were given on days 0 3 7 14 30.
Widespread immunization has dramatically altered the global landscape for the transmission of many diseases reducing morbidity and mortality1 Andre FE Booy R Bock HL Clemens J Datta SK John TJ et al.

Fda booster healthcare workers. This is true even if they recently received the Td vaccine as part of the recommended vaccine schedule for all adults in which a Td booster is given every 10 years. The regulatory and clinical details of this booster plan are contingent on FDA conducting an independent evaluation and determination of the safety and effectiveness of a third dose of the mRNA vaccines Pfizer and Moderna and CDCs Advisory Committee on Immunization Practices ACIP issuing recommendations based on a thorough review of the. The Food Drug Administration FDA on December 11 2020 granted Pfizer-BioNTech emergency use authorization EUA for its COVID-19 vaccine to be used in people age 16 and older.
Michelle Moniz said she and her colleagues learned of health care workers around the country. Hepatitis B vaccination recommendations vary by a persons age and risk factors. Phase 1A front-line healthcare workers and residents at long-term care facilities Texas continues to receive doses of the Pfizer Moderna and Johnson Johnson COVID-19 vaccines and is distributing statewide to hospitals pharmacies local health departments freestanding ERs and other clinics.
On May 10 2021 the FDA amended its original EUA for Pfizer to allow for the vaccine to be administered to people over the age of 12. The frequent-risk category includes other laboratory workers such as those doing rabies diagnostic testing spelunkers veterinarians and staff and animal-control and wildlife officers in areas where rabies is epizootic. This extra dose of a vaccine is known as a booster shot.
December 14 2020. In the Technically Speaking column in August we discussed routine hepatitis B vaccination of infants children and teens. 4th Decennial Conference March 5-9 2000.
FDA advisory panel authorizes booster dose for those 65 older Yahoo Finances Anjalee Khemlani reports the FDA advisory panels second vote regarding Covid-19. Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19. June 24 2021 -- The US.
Workers in high-density worksites or worksites with large numbers of close contacts to co-workers or customers eg restaurant workers transportation workers grocery store workers Government workers with public interactions as part of their duties eg post office workers First responders eg police fire EMT and healthcare personnel. Former Food and Drug Administration Commissioner Stephen Hahn is joining Flagship Pioneering the venture capital firm that launched Moderna as chief medical officer to lead its. Estimate of the Annual Number of Percutaneous Injuries in US.
Along with the flu and Tdap vaccine health care workers should stay up to date on their. More than 350 Indonesian doctors and healthcare workers have contracted COVID-19 despite being vaccinated with Sinovac and dozens have. Vaccination greatly reduces disease disability death and inequity worldwide.
Pending expected CDC and FDA approval. Update on Vaccinations for Dental Healthcare Personnel. Occupation and healthcare setting Panlilio AL et.
In a June 8 supply chain report the Biden administration the Department of Health and Human Services HHS and the Food and Drug Administration FDA called on Congress to give the FDA. Bulletin of the World Health Organization 2008862. Not long after the COVID-19 pandemic began Dr.
FDA approved expanded use of Sanofis Adacel Tdap vaccine for a second dose in people ages 10 through 64 years of age. It should begin with those most at risk from serious disease including older adults and frontline health and social care workers. FDA approved use of the 05 mL dose of Sanofis Fluzone Quadrivalent influenza vaccine to include children age 6 through 35 months.
But a CDC advisory panel where the FDA made the announcement still strongly backs the vaccines benefits which outweigh the rare risk for heart inflammation. This month lets review hepatitis B vaccination of adults including vaccination guidance for high-risk groups. A booster dose should be administered if the titer falls below this level.
According to the ACIP healthcare workers who havent been or are unsure if theyve been vaccinated against pertussis should get a dose of Tdap.

Fda Advisory Panel Approves Covid Boosters For Elderly High Risk People Wkbn Com
Latest Fda Panel Recommends Only Limited Use Of Pfizer Boosters

Fda Advisory Panel Rejects Widespread Pfizer Booster Shots Wciv
:strip_exif(true):strip_icc(true):no_upscale(true):quality(65)/d1vhqlrjc8h82r.cloudfront.net/09-14-2021/t_01d456ab95424780ac1e17cf9e62967e_name_image.jpg)